Functional Foods Regulation System: Proposed Regulatory Paradigm by Functional Food Center
DOI:
https://doi.org/10.31989/ffs.v3i11.1265Abstract
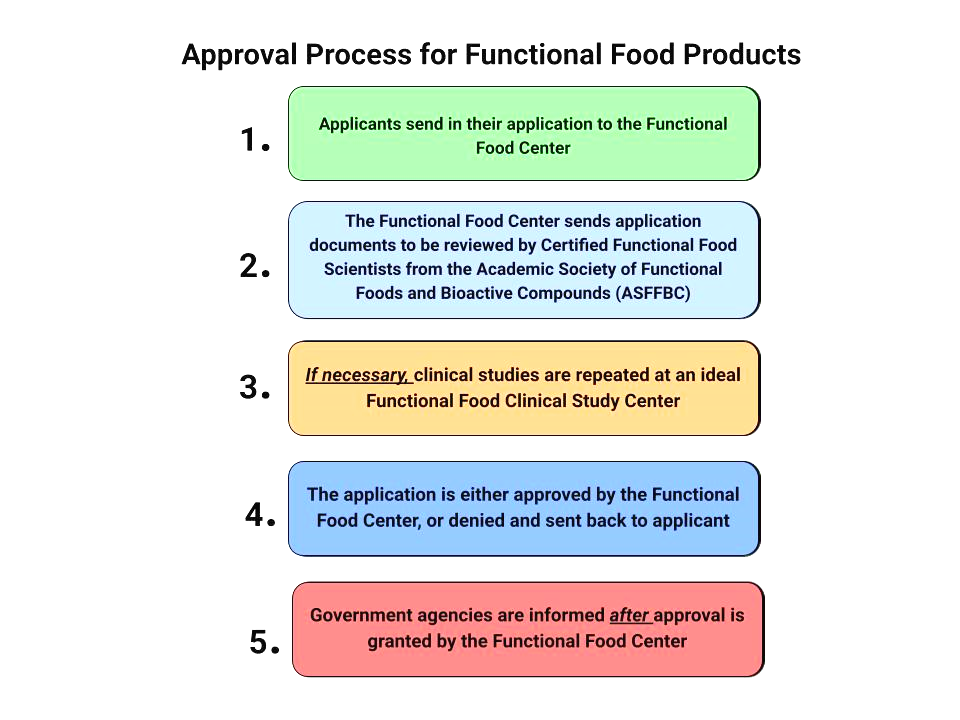
In response to the rising demand for functional foods driven by health-conscious consumers, a robust regulatory framework is imperative. The absence of specific guidelines creates uncertainties for both manufacturers and consumers. In response to the rising demand for functional foods driven by health-conscious consumers, a robust regulatory framework is imperative. The absence of specific guidelines creates uncertainties for both manufacturers and consumers. This article discusses the Functional Food Center's pioneering 17-step approval processfor functional foods, emphasizing scientific validation and transparent communication. However, challenges persist within the FDA approval system, including a protracted timeline and the lack of a dedicated category for functional foods. Drawing inspiration from the efficient kosher labeling system, the Functional Food Center could serve as a certification agency. Certified functional foods could display a designated symbol, ensuring credibility and trustworthiness. This approach streamlines the approval process, fostering innovation, ensuring consumer safety, and meeting the evolving health needs of consumers in a transparent, credible, and regulated functional food market.
Keywords:Functional Food Classification, Functional Food Regulation, Functional Food Products, Bioactive Compounds, Functional Food Safety, Kosher Labeling Model, Regulatory Paradigm.
Downloads
Published
Issue
Section
License
Copyright (c) 2023 FFS/Functional Food Science

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Authors retain the copyright of their articles and grant the Functional Food Center (FFC) and its journals the right of first publication under the terms of the Creative Commons Attribution 4.0 International License.
This license permits unrestricted use, distribution, and reproduction in any medium, including commercial use, provided the original author(s) and source are properly credited. Authors may post and share their published work freely, provided that the original publication in this journal is acknowledged.
By submitting to this journal, authors confirm that their manuscripts are original, not under consideration elsewhere, and that they hold the necessary rights to grant this license. The Functional Food Center encourages open scientific exchange and allows derivative and extended works, provided attribution to the original publication is maintained.