Development, quality and safety evaluation of a probiotic whey beverage
DOI:
https://doi.org/10.31989/ffhd.v13i7.1121Abstract
Background: This study was aimed at the development of a whey beverage enriched with a probiotic starter culture, prebiotics, vitamins, and minerals, and evaluation of microbiological, physico-chemical, and toxicological characteristics of the developed beverage.
Methods: The beverage formulation was determined based on organoleptic analysis. The assessment of microbiological and physico-chemical parameters was carried out in accordance with regulatory standards. The safety assessment of the developed drink was carried out in vivo.
Results:A beverage formulation based on whey enriched with probiotic bacteria Lactobacillus casei 1A, Lactobacillus paracasei 2A, Lactobacillus brevis 4 LB, prebiotic inulin, vitamins (A, C) and minerals (potassium iodide) was developed. The organoleptic, physico-chemical, and microbiological properties of the developed drink were determined. The quality of the beverage complied with food safety regulations, the viability of probiotic bacteria and the acidity of the beverage remained stable during storage. Acute score toxicity and allergenic properties in vivo did not reveal any physiological abnormalities and made it possible to classify the developed product as a low-hazard substance.
Conclusion:The optimal composition of a probiotic whey beverage has been developed, which can be considered as a potential product for functional nutrition.
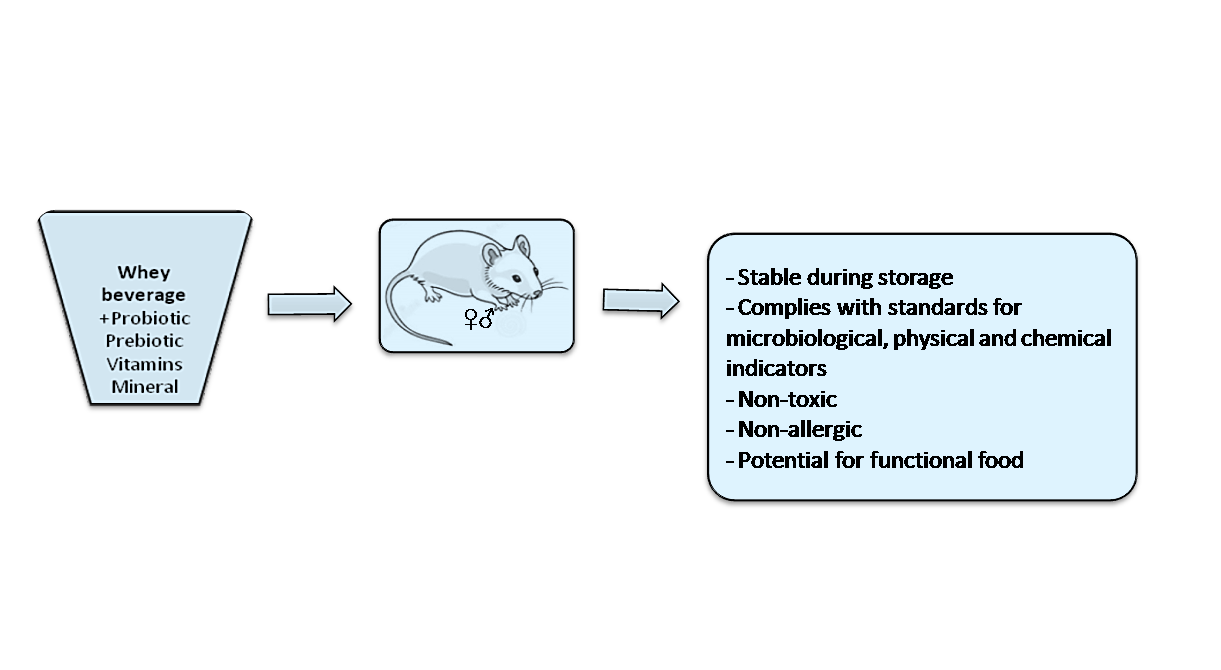
Keywords: Whey beverage, Formulation, Probiotic, Functional, Safety Assessment, Toxicity, Allergenic properties, In vivo
Downloads
Published
Issue
Section
License
Authors retain the copyright of their articles and grant the Functional Food Center (FFC) and its journals the right of first publication under the terms of the Creative Commons Attribution 4.0 International License.
This license permits unrestricted use, distribution, and reproduction in any medium, including commercial use, provided the original author(s) and source are properly credited. Authors may post and share their published work freely, provided that the original publication in this journal is acknowledged.
By submitting to this journal, authors confirm that their manuscripts are original, not under consideration elsewhere, and that they hold the necessary rights to grant this license. The Functional Food Center encourages open scientific exchange and allows derivative and extended works, provided attribution to the original publication is maintained.