Comparing the FDA's process for health claim submission and the FFC's guidelines for regulating functional foods
DOI:
https://doi.org/10.31989/ffhd.v13i9.1228Abstract
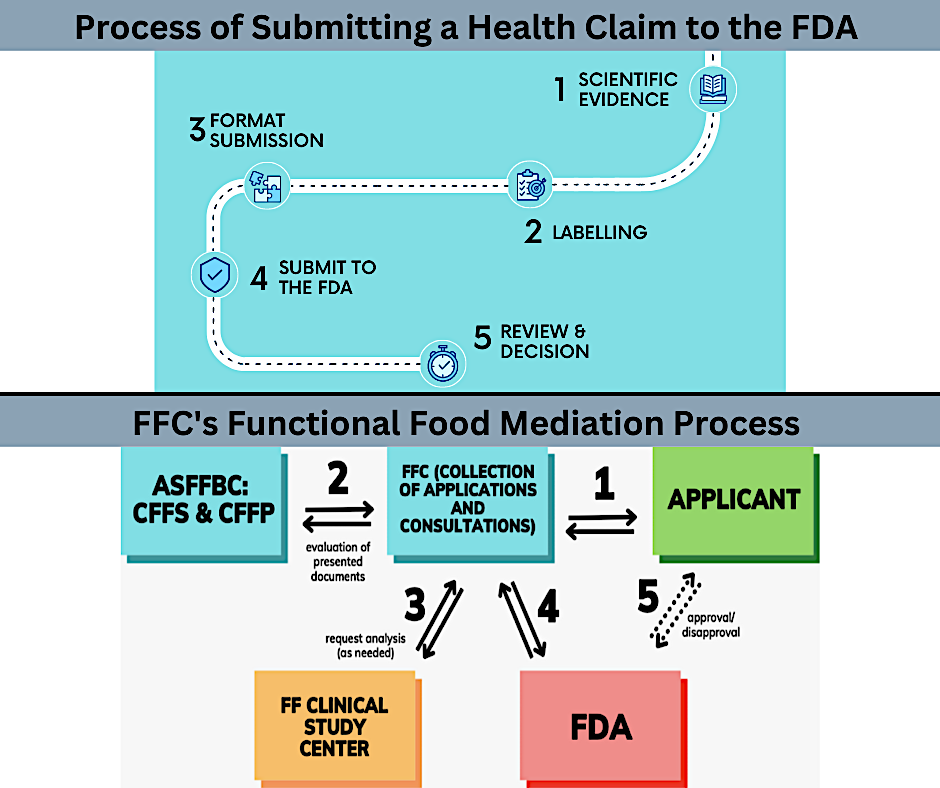
Health claims are essential for providing accurate information about the relationship between nutrients and health outcomes to consumers. They facilitate informed decision-making and support individuals in making healthy dietary choices. The U.S. Food and Drug Administration (FDA) regulates health claims to guide consumers in maintaining healthy dietary practices. Health claims on food labels help consumers understand the potential health benefits associated with specific products and make informed choices aligned with their health goals. These claims also promote transparency and trust in the food industry by providing evidence-based information. The process of submitting a health claim to the FDA involves complying with regulatory requirements and providing robust scientific evidence. Scientific evidence plays a crucial role in substantiating health claims and should be based on well-designed clinical trials, epidemiological studies, and mechanistic research. Adhering to FDA labeling requirements ensures the accurate and informative presentation of health claims on food labels. The Functional Food Center (FFC) is working on theoretical aspects of creating ideal functional food (FF) products as well as how these products should be identified with special FF labels. While the FDA focuses on evaluating health claims, the FFC emphasizes the safe and effective use of functional foods. Understanding the FDA's evaluation process and the FFC's guidelines is crucial for researchers, food manufacturers, and policymakers to navigate the regulatory landscape and promote informed consumer choices. Compliance with regulatory guidelines, adherence to scientific standards, and clear communication of health claims contribute to a more informed and health-conscious society.
Keywords: Health claims, health outcomes, regulatory requirements, functional foods, health benefits
Downloads
Published
Issue
Section
License
Any manuscripts or substantial parts of it, submitted to the journal must not be under consideration by or previously published in any other journal or citable form. Authors are required to ensure that no material submitted as part of a manuscript infringes existing copyrights or the rights of a third party. In submitting one's article in any form, the author has assigned the FFC publishing rights and has agreed to an automatic transfer of the copyright to the publisher. This is so that the FFC may create print option journals, for example, at the FFC’s discretion. If the author wishes to distribute their works by means outside of the FFC, for example within their community, they will have to place a request.
Correspondence concerning articles published in Functional Foods in Health and Disease is encouraged. While derivative works (adaptations, extensions on the current work, etc.) are allowed, distribution of the modified material is not allowed without permission from the FFC.