Chemical, microbial and safety profiling of a standardized Withania somnifera (Ashwagandha) extract and Withaferin A, a potent novel phytotherapeutic of the millennium
DOI:
https://doi.org/10.31989/ffhd.v13i2.1071Abstract
Background:Withania somnifera (L.) Dunal, popularly known as Ashwagandha, is an ethnomedicinal plant with multiple pharmacotherapeutic applications. The diverse medicinal properties of the plant are largely due to the presence of withanolides, a group of C28 ergostane based steroidal lactones, with several sites of unsaturation and oxygenation. Withaferin A, a major with anolide present in Ashwagandha plant accounts for its emerging new roles to treat cancer, arthritis, inflammatory responses, immunomodulatory properties, and neuronal disorders. The root and leaf extracts are specifically important constituent materials for the development of phytotherapeutics, mostly intended for oral consumption. Several studies have been carried out to delineate the toxic manifestations of the extract for human consumption.
Objective:Establish the broad-spectrum safety of W-ferinAmax ashwagandha (WFA).
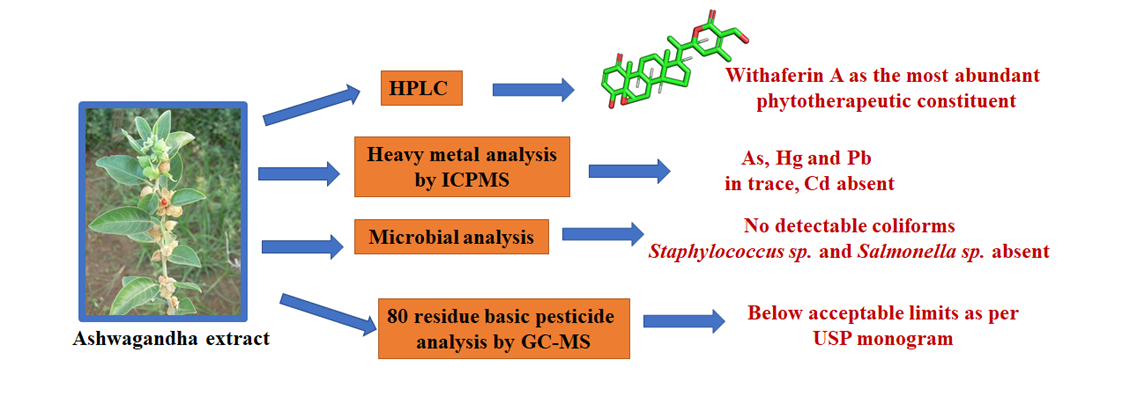
Study: This investigation demonstrated a novel, standardized W-ferinAmax ashwagandha (WFA) extraction technology from the whole herb of Withania sominfera, conducted HPLC analysis to identify the constituents, detected the heavy metals, microbiological contaminants, pesticides contaminants, and safety profile.
Results:A novel extraction technology was employed to obtain WFA from the whole plant of Withania sominfera. HPLC analysis revealed that WFA contains a total of 15.4% Withanolides. In particular, Withaferin A, Withanoside IV, and Withanolide A contents were 6.469%, 1.022%, and 0.073%, respectively. The extract contained only 0.403 ppm of heavy metals out of which traces of arsenic, mercury and lead were detected, and cadmium was absent. USP recommended 80 residue basic pesticide screen indicated that the extraction was safe for human consumption. It was also found to be free from pathogenic microbes as assessed by the absence of E. coli and other coliforms, Salmonella and Staphylococcus species.
Conclusion: The data generated cumulatively indicated that WFA is safe for further downstream processing to and for human consumption.
Keywords: Ashwagandha, Withaferin A, phytotherapeutics, material safety; heavy metals; pesticides
Downloads
Published
Issue
Section
License
Any manuscripts or substantial parts of it, submitted to the journal must not be under consideration by or previously published in any other journal or citable form. Authors are required to ensure that no material submitted as part of a manuscript infringes existing copyrights or the rights of a third party. In submitting one's article in any form, the author has assigned the FFC publishing rights and has agreed to an automatic transfer of the copyright to the publisher. This is so that the FFC may create print option journals, for example, at the FFC’s discretion. If the author wishes to distribute their works by means outside of the FFC, for example within their community, they will have to place a request.
Correspondence concerning articles published in Functional Foods in Health and Disease is encouraged. While derivative works (adaptations, extensions on the current work, etc.) are allowed, distribution of the modified material is not allowed without permission from the FFC.